Cent_Eur_Ann_Clin_Res 2020, 2(1), 53; doi:10.35995/ceacr2010053
Endoped Abstract
Short Stature Due to Isolated Growth Hormone Deficiency—Case Report
Department of Endocrinology, Rosana Medical Clinic Bucharest, Bucharest 061973, Romania; mihaelaviorica@gmail.com
How to cite: Popescu, M.V. Short Stature Due to Isolated Growth Hormone Deficiency—Case Report. Cent. Eur. Ann. Clin. Res. 2020, 2(1), 53; doi:10.35995/ceacr2010053.
Received: 26 October 2020 / Accepted: 14 November 2020 / Published: 17 November 2020
Abstract
:Short stature is a common cause for addressing the endocrinologist. Growth hormone deficiency is estimated to occur in 1:4000 to 1:10,000 of small stature cases [1]. Identifying and treating these children is of high importance given the psycho-social implication of small stature. I present the case of a male teenager ages 15 years 10 months old who responded well to treatment, achieving the target genetic stature.
Keywords:
auxology; genetic height target; GH deficiency; short statureBackground and Aims
A boy of 15 years and 10 months is taken to the clinic for initial endocrinological evaluation, having a height of 158.5 cm (−2.18 SDS). From the history presented by the GP doctor, we note a linear increase of 7 cm in the last 2 years and 1.5 cm in the last year, with a decrease in the growth rate (−1.83 SDS). The genetic target height is 178.5 cm (+0.21 SDS). The difference in relation to the genetic target height is −2.39 SDS. Pubertal development: Tanner G2P2. Bone age at initial evaluation: 13 years and 6 months; 2 years and 4 months late compared to the chronological age. Insulin growth factor 1 (IGF-1) at the lower limit of normal. The functional reserve of the somatotropic axis was evaluated: insulin and clonidine Growth hormonr (GH) stimulation tests—negative (GH peak to Insulin 2U = 0.108 ng/mL and GH peak to clonidine 0.15 mg/m2 = 4.310 ng/mL) [2,3].
Somatropin 0.7 mg/m2 body surface area treatment was initiated in CF2 Clinical Hospital Bucharest so that he could reach the desire height.
Materials and Methods
Growth charts, insulin and clonidine GH stimulation tests, IGF-1 dosing, hand X-ray. The initiation of somatropin treatment was made according to the criteria of the: Romanian therapeutic protocol for Somatropinum in children and in the transition period: height deficit between −2 and −2.5 SD and accentuation of the stature deficit by 0.5 SD per year as well as height deficit between −2 and −2.5 SD and height smaller with 1.6 SD below the genetic target [4,5].
Results and Conclusions
The auxological evolution, bone age and IGF1 values during the treatment are shown in Table 1.
Given that the desired height has been reached, the treatment was stopped at the age of 17 and 4 months. The peculiarity of this case consists in initiating somatropin in older child, with a good response to the low dose of Somatropin and reaching the target height, as illustrated in Figure 1.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- Stanley, T. Diagnosis of Growth Hormone Deficiency in Childhood. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, A.; Allen, D.B. Growth Hormone Treatment for Growth Hormone Deficiency and Idiopathic Short Stature: New Guidelines Shaped by the Presence and Absence of Evidence. Curr. Opin. Pediatr. 2017, 29, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Clayton, P.E.; Cuneo, R.C.; Juul, A.; Monson, J.P.; Shalet, S.M.; Tauber, M. Consensus statement on the management of the GH-treated adolescent in the transition to adult care. EJE 2005, 152, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Pascanu, I.; Pop, R.; Barbu, C.G.; Dumitrescu, C.P.; Gherlan, I.; Marginean, O.; Preda, C.; Procopiuc, C.; Vulpoi, C.; Hermanussen, M. Development of synthetic grows charts for Romanian population. Acta Endocrinol. 2016, XII, 309–318. [Google Scholar]
- List of Therapeutic Protocols Approved by Ordinul MS/CNAS no. 1301/500/2008 with Subsequent Amendments and Completions. Available online: www.cnas.ro (accessed on 10 October 2020).
Figure 1.
Height evolution.
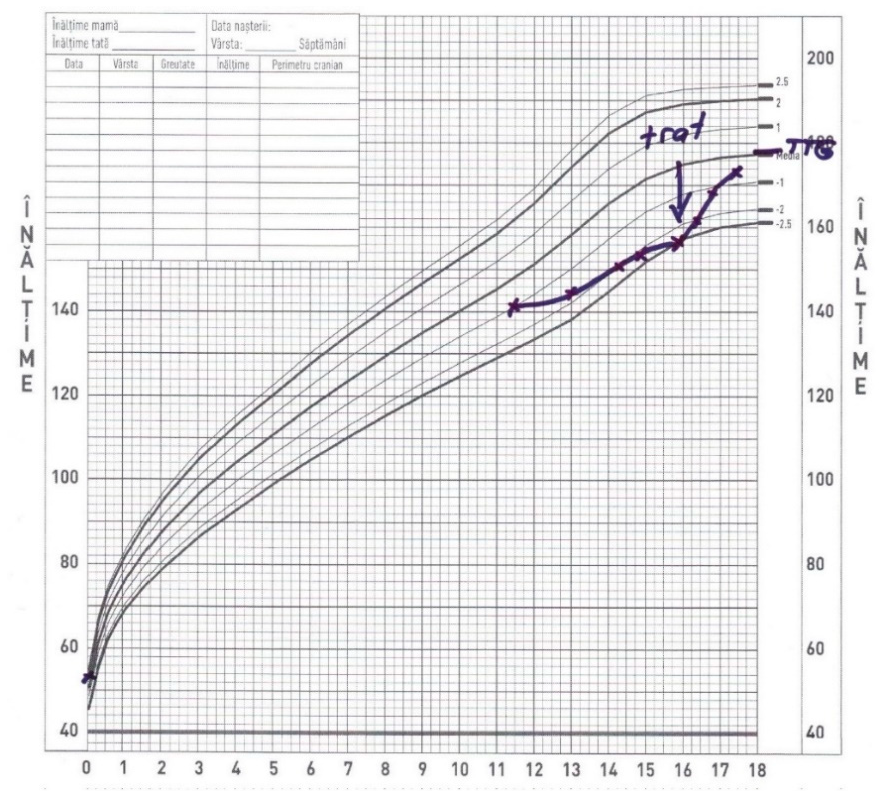
Table 1.
Monitoring of auxological parameters, IGF-1 and bone age.
| Chronological Age | Height-cm (SD) | IGF-1 (ng/mL) | Bone Age | Somatropin Dosage |
|---|---|---|---|---|
| 15 years 10 months | 158.5 cm (−2.18 SD) | 211 (N:211–512) | 13 years 6 months | 0.7 mg/m2 |
| 16 years 4 months | 163.3 cm (−1.73 SD) | 333 (N:57–426) | 14 years 0 months | 0.7 mg/m2 |
| 16 years 10 months | 169 cm (−1.05 SD) | 489 (N:57–426) | 0.7 mg/m2 | |
| 17 years 4 months | 174.5 cm (−0.31 SD) | 412 (N:57–426) | 15 years 6 months |
© 2020 Copyright by the authors. Licensed as an open access article using a CC BY 4.0 license.

